The Nucleus of Neurons
If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com or the author: bensonbush@mail.weber.edu
The Nucleus
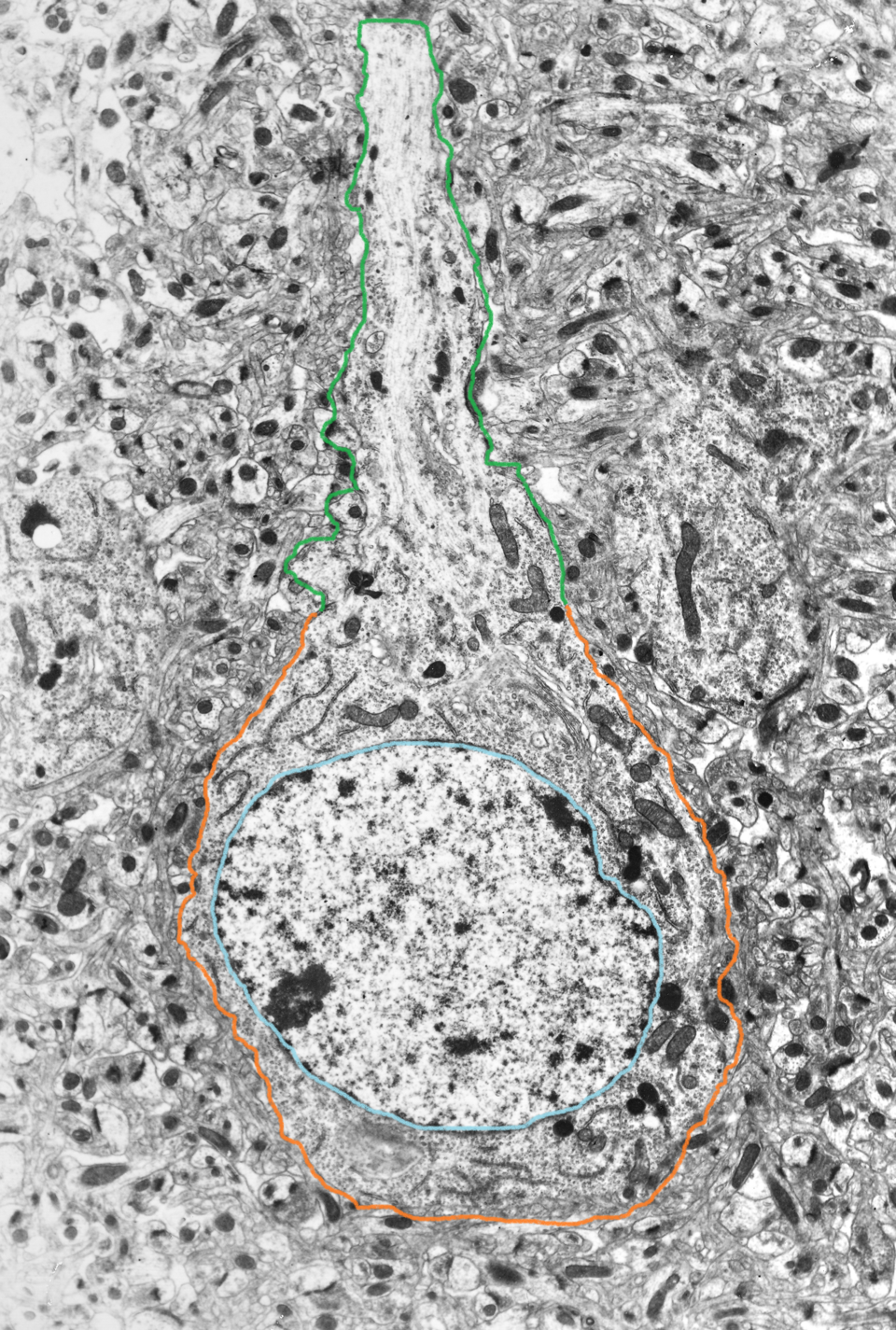
The nucleus is an organelle located in the center of the neuronal cell body, or soma. Its purpose is to house the genetic information used in the development and maintenance of the cell. Transcription, the conversion of DNA to its RNA form (a primary transcript), followed by editing of the primary transcript into messenger RNA, occurs here.
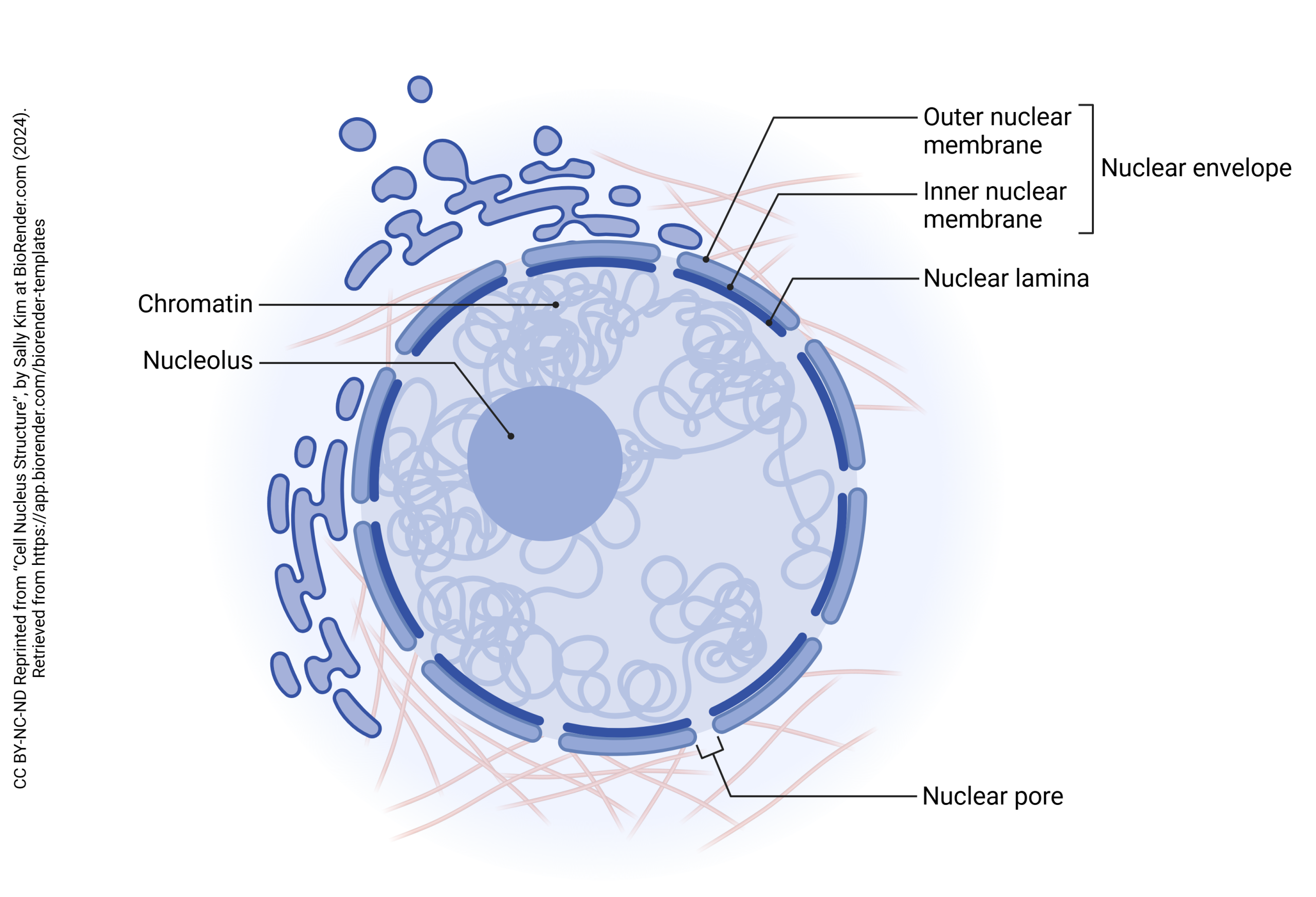
The nucleus is surrounded by a nuclear envelope. This is made up of the outer nuclear membrane, inner nuclear membrane, and nuclear lamina. The nuclear envelope is continuous with the endoplasmic reticulum, which is the organelle involved in the process of protein synthesis.
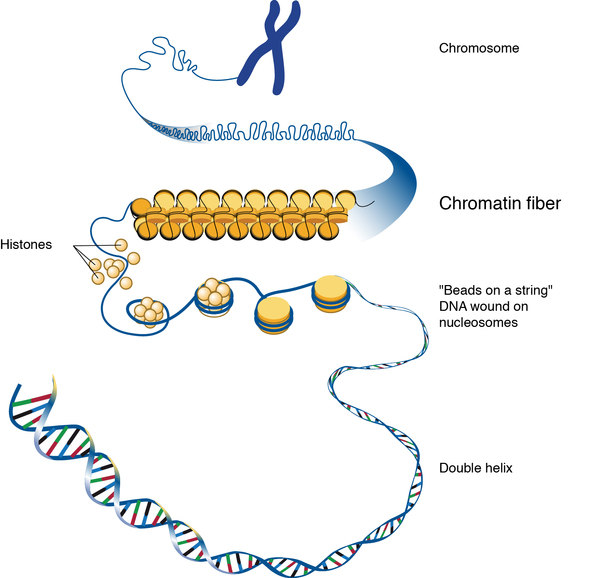
Within the envelope and suspended in nucleoplasm are coils chromatin, which is made up of strands of DNA wrapped around histones. Chromatin can be categorized into two types: heterochromatin and euchromatin. Heterochromatin is very condensed with little to no important genes that aren’t activated as often for transcription, while euchromatin is less condensed and contains more useful genes that are transcribed often (Tamaru, 2018).
Nestled in the center of the nucleus is the nucleolus which is a crucial site for neuronal development and long-term maintenance of stress responses in mature neurons through the production and assembly of ribosomes to be sent out to the cytoplasm through nuclear pores (Fawcett, 1917; Hetman & Pietrzak, 2012). Dysfunction here may be linked to disrupted neurodevelopment leading to apoptosis or neurodegeneration in diseases like Alzheimer’s due to the slowing of ribosome biogenesis, which will lead to less ribosome transcription happening, decreasing protein synthesis over time.
[amplify on histones and methylation?]
The Cell Cycle
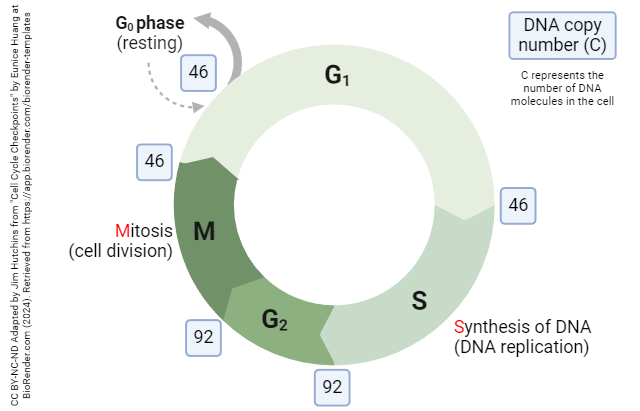
Cellular replication occurs in the cell in a cyclic pattern across the cell’s lifetime (Alberts et al., 2002). The stages of this cycle are G1, S, G2, and M. The G1 stage, or the first “gap” stage, is where cells begin the process of cell replication by doubling the amount of proteins and organelles to get ready for cell division. Then, in the S stage, or synthesis stage of the cell cycle, synthesis of DNA occurs where DNA is replicated. Following the S stage, the second gap stage, G2, provides a similar time period as G1 for cells to continue synthesizing new proteins and organelles. Finally, the cell will divide at the M stage, or mitosis, and the process begins anew. If conditions are not favorable for cell reproduction, it may take longer to enter G1 again after dividing in the M stage. If this occurs, they enter G0, or a resting phase. Specific types of cells, such as neurons, do not typically replicate, so they are permanently in the G0 phase, or resting phase.
Lack of Replication in Neurons
(under construction)
- Neurons do not replicate, use information from Sotnikov et al. 2010 paper
- Neuron development stops at G0
- Possibly replicate?
- Hippocampal neurogenesis
- Stem cell research information here
- https://www.proquest.com/docview/207550201?pq-origsite=gscholar&fromopenview=true&sourcetype=Scholarly%20Journals
Media Attributions
- Obj05-05-U13-078-axonal-transport-of-neuropeptides-1
This is a first draft which has not been edited. If you have questions, or want to help in the writing or editing process, please contact the author: jacksonstringham@mail.weber.edu
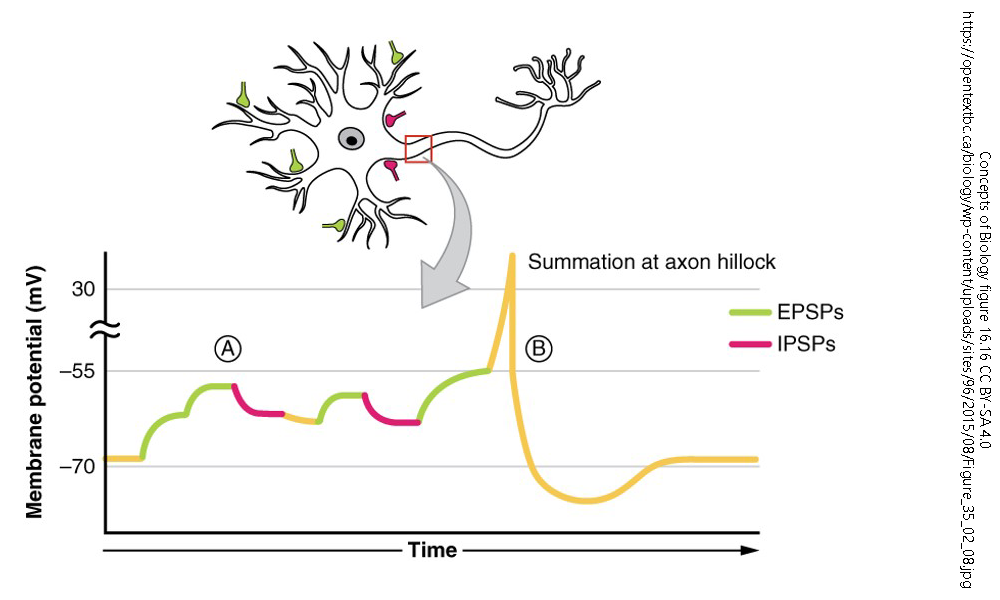
Neurons communicate with one another through electrical and chemical signals. One key aspect of neuronal communication is the integration of multiple synaptic inputs to determine whether an action potential will be generated. This process is governed by two fundamental mechanisms: temporal summation and spatial summation. These summation processes influence how signals are processed within neural circuits and ultimately determine the response of the postsynaptic neuron.
Graded Potentials and Summation
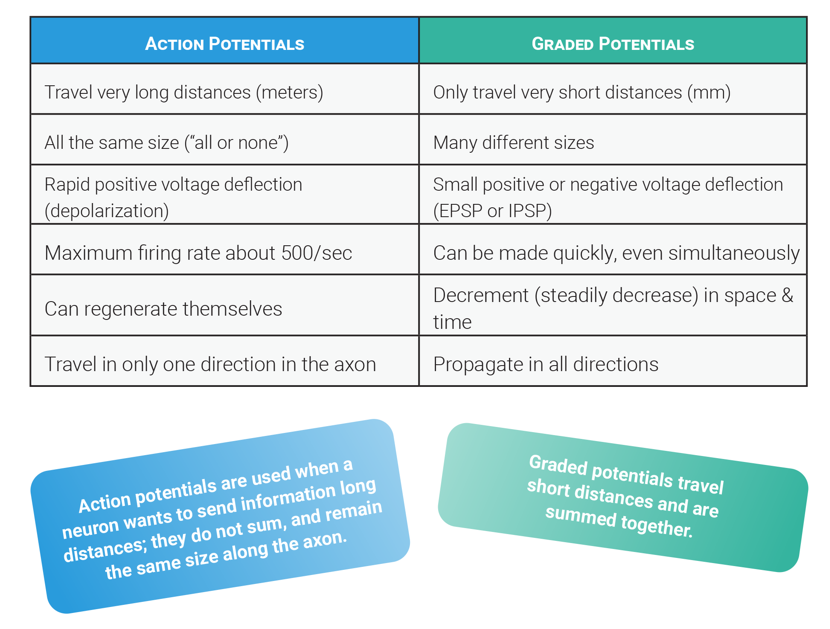
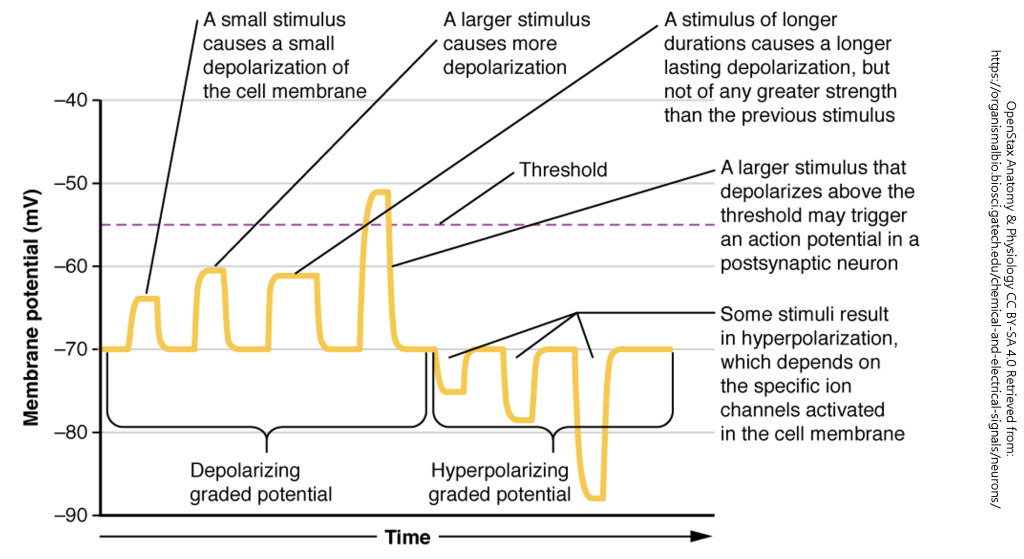
Neurons receive input from presynaptic neurons via synapses, where neurotransmitters bind to receptors, leading to the generation of graded potentials. These are small, localized changes in the membrane potential that can be either excitatory or inhibitory. Unlike action potentials, graded potentials vary in magnitude and decay over distance.
Summation refers to the additive effect of multiple graded potentials occurring in close temporal (time) or spatial (space). If the summation of excitatory postsynaptic potentials (EPSPs) reaches the threshold at the axon hillock, an action potential is generated. Conversely, inhibitory postsynaptic potentials (IPSPs) can counteract EPSPs, preventing an action potential from occurring.
Temporal Summation
Temporal summation occurs when multiple EPSPs or IPSPs from a single presynaptic neuron arrive at the postsynaptic membrane in rapid succession. Because graded potentials last for a few milliseconds, subsequent signals arriving before the previous ones decay can sum together, increasing the likelihood of reaching the threshold potential.
The Steps of Temporal Summation:
- A single presynaptic neuron releases neurotransmitters multiple times within a short period.
- The resulting graded potentials overlap and build on each other.
- If EPSPs summate sufficiently, they depolarize the membrane to the threshold, triggering an action potential.
- If IPSPs summate, they hyperpolarize the membrane, reducing the likelihood of an action potential.
Resonance?
X
Article example:
Izhikevich, E. M., Desai, N. S., Walcott, E. C., & Hoppensteadt, F. C. (2003). Bursts as a unit of neural information: selective communication via resonance. Trends in neurosciences, 26(3), 161-167. https://www.cell.com/trends/neurosciences/fulltext/S0166-2236(03)00034-1
Spatial Summation
Spatial summation occurs when graded potentials from multiple presynaptic neurons arrive at different locations on the postsynaptic neuron simultaneously. The combined effect of these inputs determines whether the neuron reaches the threshold for an action potential.
The Steps of Spatial Summation:
- Multiple presynaptic neurons release neurotransmitters onto different regions of the postsynaptic membrane.
- If multiple EPSPs converge, their combined depolarization may reach the threshold.
- If IPSPs are also present, they may counteract the EPSPs, reducing the likelihood of reaching the threshold.
- The net effect of all incoming signals determines whether the postsynaptic neuron fires an action potential.
Functional Importance of Summation
Temporal and spatial summation are essential for neural processing and integration. These mechanisms allow neurons to:
- Filter and prioritize incoming signals.
- Integrate information from multiple sources.
- Regulate neuronal firing rates and patterns.
- Facilitate complex functions such as sensory perception, motor control, and cognition.
For example, in the spinal cord, summation plays a crucial role in reflex arcs, where multiple sensory inputs influence motor output. Similarly, in the brain, summation underlies decision-making processes, where excitatory and inhibitory inputs shape neuronal activity.
Examples in Visual and Pain Processing
Temporal and spatial summation are particularly important in sensory systems like visual and pain processing. In the visual system, spatial summation enables the integration of light stimuli from multiple photoreceptors in the retina, enhancing visual perception under low-light conditions. Temporal summation in vision helps detect motion by integrating successive visual stimuli, allowing the brain to perceive smooth movement rather than discrete flashes of light.
In pain processing, spatial summation plays a critical role in determining the intensity of a painful stimulus. When multiple nociceptive (pain) neurons are activated simultaneously, their signals combine to amplify the perception of pain. Temporal summation of pain signals occurs when repeated stimulation over time leads to an increased perception of pain, a phenomenon known as wind-up, which contributes to chronic pain conditions. These summation mechanisms allow the nervous system to assess and respond appropriately to painful stimuli, influencing pain perception and pain management strategies.
Conclusion
Temporal and spatial summation are fundamental mechanisms by which neurons integrate multiple synaptic inputs. Temporal summation enhances signal strength through rapid successive inputs, while spatial summation integrates signals from multiple sources. The interplay of these mechanisms determines neuronal firing and is essential for complex neural processing. Understanding summation provides insight into how the nervous system interprets and responds to stimuli, ultimately shaping behavior and cognition.